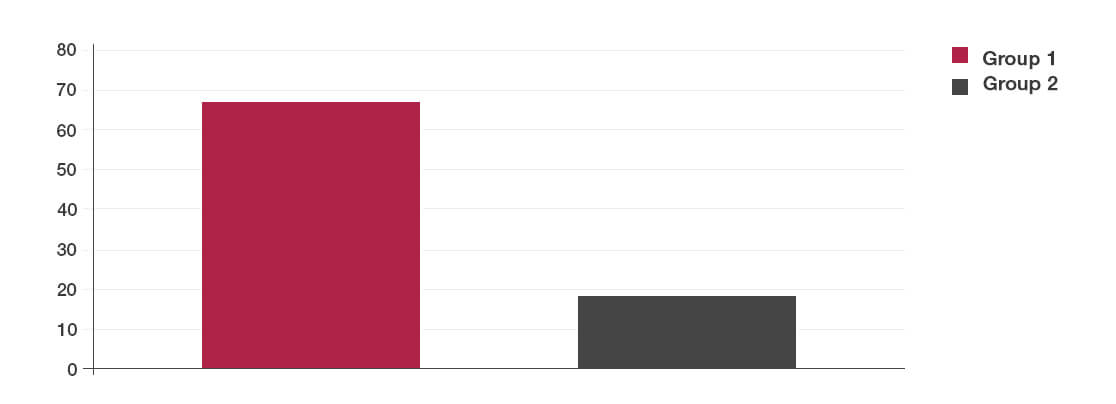Cambridge Clostridium difficle Study
Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea
Aim
This randomised, double-blind, placebo controlled study was designed to provide evidence that supplementing with Lab4 probiotics while taking antibiotics can prevent the incidence of antibiotic associated C. difficile diarrhoea (CDAD).
Method
- The study was carried out at Addenbrooke’s Hospital in Cambridge.
- 138 elderly patients requiring antibiotic therapy were divided into two equal groups.
- Group 1 received daily one placebo capsule with their prescribed antibiotics for 20 days.
- Group 2 received daily one capsule of 25 billion Lab4 probiotics in conjunction with their prescribed antibiotics for 20 days.
- The efficacy of Lab4 probiotics in the prevention of CDAD was assessed by recording bowel habit and analysing faecal samples.
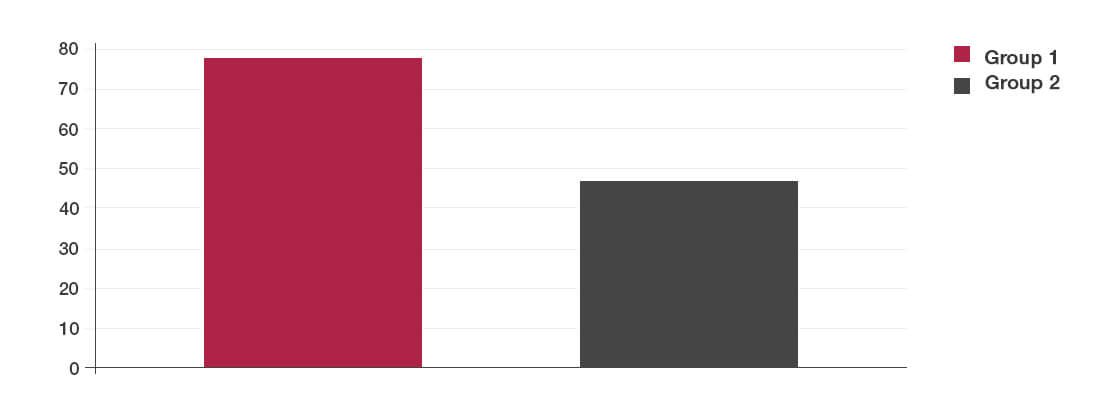
Results
- The patient group given Lab4 probiotics experienced a lower incidence of Clostridium difficile diarrhoea compared to the placebo group.
- This effect was due to a reduction in the presence of the Clostridium difficile toxin in the Lab4 probiotic group.
Conclusion
Supplementation with Lab4 probiotics can reduce the incidence of C. difficile diarrhoea in hospitalised patients.
Reference
Plummer S et al 2004.
Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea.
International Microbiology, 7:59-62

 Available from the 8th March to the 19th March on our three most popular women’s probiotics.
Available from the 8th March to the 19th March on our three most popular women’s probiotics.  Discount added automatically at checkout for you.
Discount added automatically at checkout for you.