Safety in Newborns Study
Dietary Supplementation with Lactobacilli and Bifidobacteria Is Well Tolerated and Not Associated with Adverse Events during Late Pregnancy and Early Infancy
Aim
The study evaluated the safety of Lab4b probiotic supplementation in two potentially vulnerable populations – mothers during the last month of pregnancy and their healthy newborn babies during the first six months of life.
Method
- The study was carried out at Swansea University Medical School.
- 454 mother/baby pairs took part in the trial.
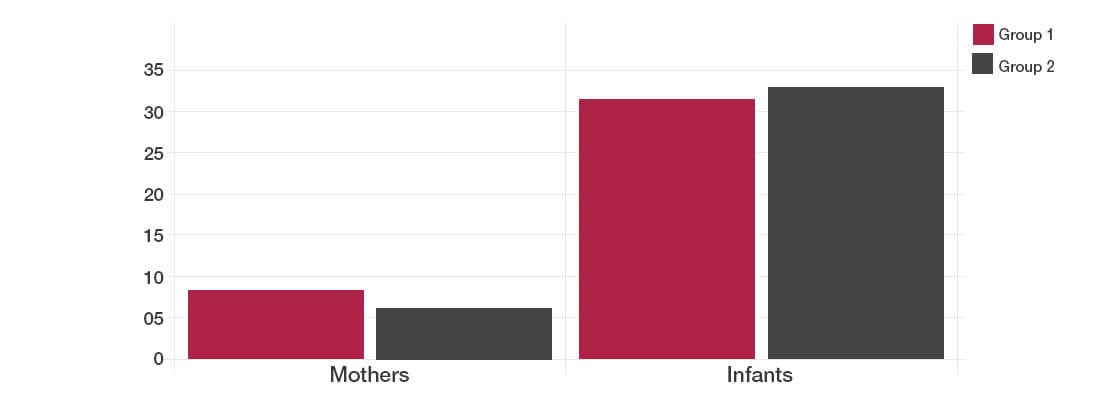
- Group 1: The mothers took daily a placebo during the last month of pregnancy and then gave the same preparation to their newborn babies every day for 6 months following birth
- Group 2: The mothers took 10 billion of the Lab4b probiotic per day during the last month of pregnancy and then gave the same probiotic to their newborn babies every day for 6 months following birth.
- All adverse health events were recorded in both the mothers and babies to determine whether there were any differences between the Lab4b probiotic and placebo group.
Results
No differences were found in total adverse events either in the mums-to-be or the babies between the Lab4b probiotic group and the placebo group.
Safety assessment
Conclusion
This study confirms that the use of Lab4b probiotic is perfectly safe for mums-to-be and their healthy newborn babies.
Reference
Allen SJ et al 2010. Dietary supplementation with Lactobacilli and Bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy.
Journal of Nutrition 140:483-488

 Available from the 8th March to the 19th March on our three most popular women’s probiotics.
Available from the 8th March to the 19th March on our three most popular women’s probiotics.  Discount added automatically at checkout for you.
Discount added automatically at checkout for you.