The Sheffield IBS Trial
Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study
Aim
This randomised, double-blind, placebo controlled study investigated the effect of supplementation with Lab4 probiotics to significantly reduce the symptoms of IBS.
Method
- The study was carried out at The University of Sheffield.
- 52 people diagnosed with IBS by the Rome II criteria were divided into two groups.
- Group 1 received daily a placebo preparation for 8 weeks.
- Group 2 received daily 25 billion Lab4 probiotics for 8 weeks.
- The IBS symptoms were assessed every two weeks during the study period and again at 10 weeks – two weeks after stopping the probiotics or placebo.
- The total IBS symptoms measured included:
- Number of days with pain
- Level of abdominal pain
- Bloating
- Quality of life
- Satisfaction with bowel habit
Results
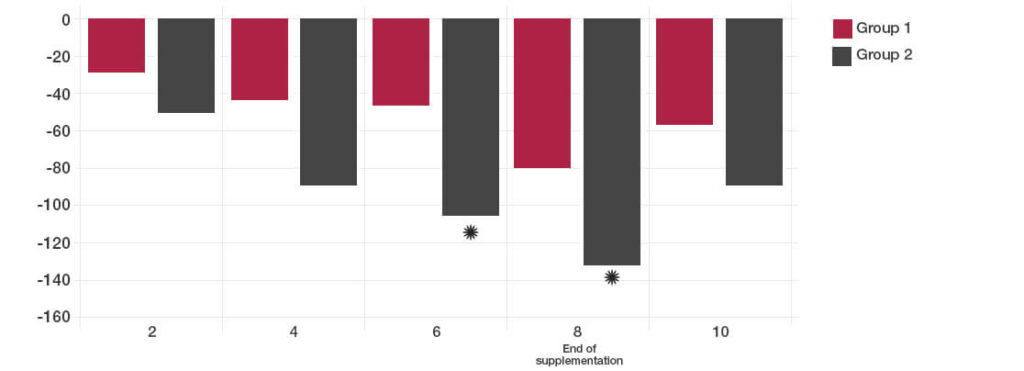
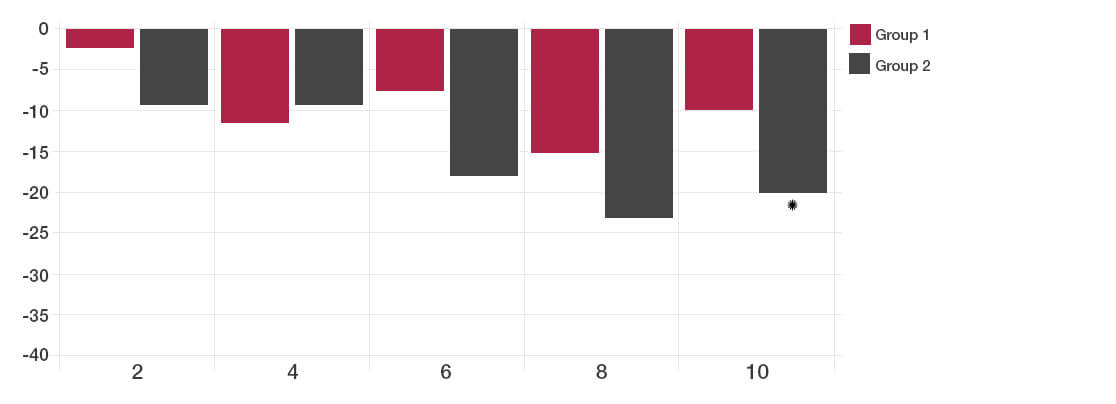
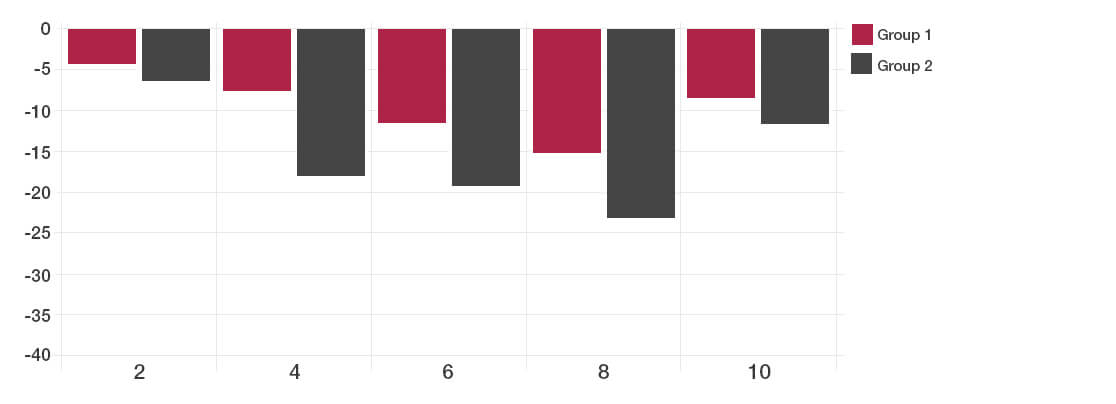
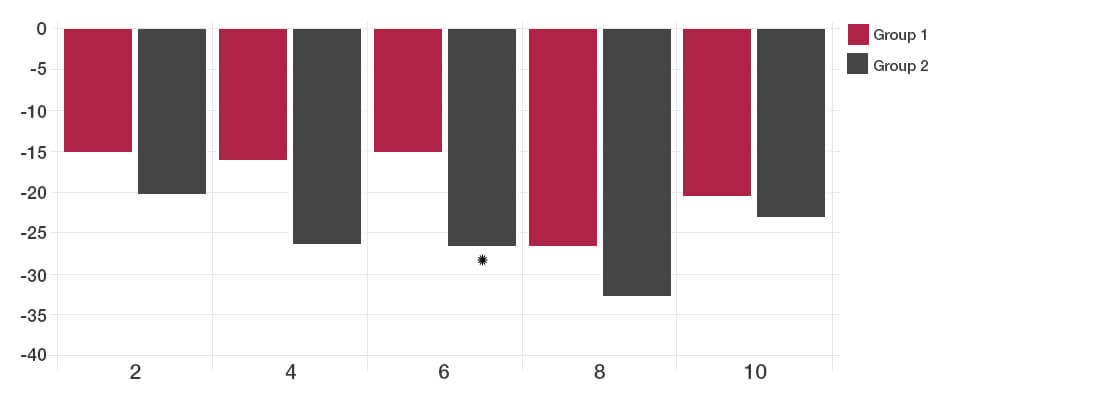
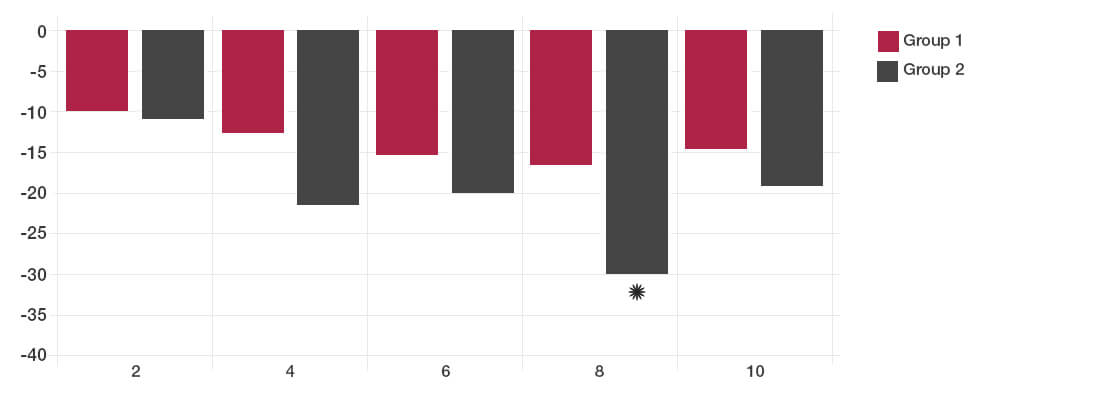
The participants taking the Lab4 probiotics showed significant improvement in Total IBS symptoms compared to placebo (*P<0.05).
By two weeks after the intervention, no significant difference could be detected between the probiotic and placebo groups.
Total IBS symptoms during follow up time (weeks)
The reductions in days with pain and bloating were achieved with the Lab4 probiotic group.
Satisfaction with bowel habit and quality of life were significantly improved in the Lab4 probiotic group (reduction in the symptom severity scores).
Conclusion
The Lab4 probiotics significantly reduced total symptoms and improved quality of life in diagnosed IBS sufferers. Continued supplementation was considered necessary to sustain this improvement.
Reference
Williams EA et al 2008. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study.
Alimentary Pharmacology & Therapeutics, 29: 97-103

 Available from the 8th March to the 19th March on our three most popular women’s probiotics.
Available from the 8th March to the 19th March on our three most popular women’s probiotics.  Discount added automatically at checkout for you.
Discount added automatically at checkout for you.