Swansea Baby Trial
Probiotics in the prevention of eczema: a randomized controlled trial
Aim
This large randomised, double blind, placebo controlled study was designed to evaluate whether Lab4b probiotics given during infancy could prevent allergy in children.
Method
- The study was carried out at Swansea University Medical School.
- 454 mother/baby pairs took part in the trial.
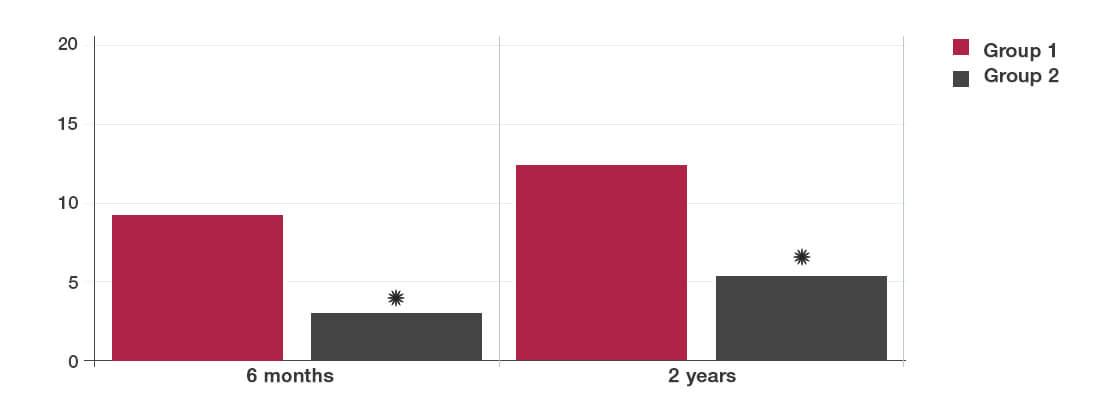
- Group 1: The mothers took daily a placebo during the last month of pregnancy and then gave the same preparation to their newborn babies every day for 6 months following birth.
- Group 2: The mothers took 10 billion of the Lab4b probiotic per day during the last month of pregnancy and then gave the same probiotic to their newborn babies every day for 6 months following birth.
- Skin prick tests were carried out on both the Lab4b probiotic and placebo infant groups to detect allergic reaction to the most common allergens, including cow’s milk, egg, house dust mite and pollen.
- Atopic eczema was defined as eczema with one or more positive SPT’s
Results
- The babies given the Lab4b probiotics were 57% less likely to develop atopic eczema than those receiving the placebo (*P<0.05).
- The babies given Lab4b were 44% less likely to develop allergic reaction to common allergens, including pollen, cow’s milk, egg and house dust mite (*P<0.05) .
Conclusion
The primary author, Prof. Steve Allen, concluded the following key message from the trial: ‘Lactobacilli and Bifidobacteria administered to pregnant women and infants aged 0-6 months prevented atopic sensitization and atopic eczema.’
Reference
Allen SJ et al 2014. Probiotics in the prevention of eczema: a randomised controlled trial.
Archives of Disease in Childhood 99(11): 1014–1019

 Available from the 8th March to the 19th March on our three most popular women’s probiotics.
Available from the 8th March to the 19th March on our three most popular women’s probiotics.  Discount added automatically at checkout for you.
Discount added automatically at checkout for you.